EU MDR & IVDR: Medical Device Regulations
$40.00 US
Course duration: Allow 2 to 4 hours to complete this course.
- Satisfaction Guaranteed
- No Hassle Refunds
- Secure Payments
Description
Learn important EU MDR & IVDR compliance requirements detailed in the EU MDR – European Union Medical Device Regulation (2017/745) and In Vitro Diagnostic Medical Devices Regulation (2017/746) in our fully-online Certificate GMP course.
EU MDR & IVDR Compliance Training – Fully Online Certificate Course
Gain essential knowledge of EU regulatory requirements for medical devices and in vitro diagnostics in our online GMP Certificate Course, which overview compliance requirements for the following EU regulations for medical devices:
-
EU Medical Device Regulation (MDR) 2017/745
-
EU In Vitro Diagnostic Medical Devices Regulation (IVDR) 2017/746
The EU MDR and IVDR are now fully in force. All manufacturers, importers, and authorised representatives are expected to comply.
What You’ll Learn
This self-paced course (approx. 2–4 hours) introduces key compliance topics:
-
Key differences between EU MDR & EU IVDR and the previous EU directives these regulations replaced (e.g., replacing MDD, AIMD, IVDD)
-
Risk classification changes and their impact
-
Notified Body involvement and Conformity Assessments
-
General Safety & Performance Requirements (Annex I)
-
EUDAMED database updates and UDI system expectations
-
PRRC responsibilities (Person Responsible for Regulatory Compliance)
-
Post-market surveillance and lifecycle risk management
-
Clinical evaluation and QMS alignment with ISO 13485
-
Risk management aligned with ISO 14971
Who Should Enroll in EU MDR & IVDR online training.
This course is designed to support professionals involved in medical device manufacturing, importing, regulatory affairs & compliance inspections, including:
-
Quality Managers
-
Regulatory Affairs personnel
- Site Inspectors
-
Responsible Persons
-
Importers and Authorised Representatives
-
Developers of medical software and IVDs
- Other stakeholders who need to better understand the guidance
This course is for education purposes only.
- Note: Learners must still read these regulations in entirety.
- Learners should also have appropriate onsite Supervision.
- This course is best supplemented with other training covering medical device manufacturing standards, including ISO standards for medical devices.
Benefits of this course
-
Provides a strong foundation in the MDR and IVDR frameworks
-
Supports audit readiness and regulatory understanding
-
Helps build a culture of quality and compliance
-
Includes a Final Assessment and Certificate of Completion
Additional Info
-
100% online and available 24/7
-
Bundled course options available (including ISO 13485, ISO 14971, CAPA, complaints handling, etc.)
-
Discounts for group training or multi-course packages (click on Bundles or contact us for training large groups of personnel)
Reminder
This course is for educational purposes only and does not replace reading the full MDR/IVDR regulations or product-specific training. You are still responsible for understanding and applying the regulations relevant to your role and jurisdiction.
Additional Course Details
Course Agenda (Overview)
This Certificate Training Course introduces key elements of EU MDR & EU IVDR compliance requirements. Topics introduced include:
- What is EUDAMED and how is it used?
- What is the NANDO database used for?
- EU Notified Bodies (NBs) vs UK Notified Bodies
- Increased monitoring of NBs by Government Authorities to ensure product safety & performance
- What new product risk classifications (reclassifications in the EU MDR) mean regarding greater involvement of Notified Bodies (NBs)
- Annex I – General Safety & Performance Requirements (overview of key concepts)
- Person Responsible for Regulatory Compliance (PRRC)
- Stronger focus on Quality Management Systems (QMS) and risk management principles for product safety/patient safety
- EU UDI system – Differences in the EU’s UDI system compared to other countries such as the USA (FDA), TGA etc.
- Responsibilities of key players in the medical device supply chain
- Legal liability for product safety and EU MDR compliance
- Conformity Assessment routes and processes
- CE marks/CE marking
- Preparing for a Conformity Assessment
- EU MDR training resources for manufacturers
- Ongoing medical device safety monitoring (post-market surveillance requirements)
Background to updated EU regulations for medical devices
- Transitional periods for certain IVDR products were put in place to avoid medical device supply chain disruptions.
- The EU MDR and EU IVDR are in force, but there are some remaining transition periods for IVDR compliance.
- Extended transitional timelines for compliance with EU IVDR (the Australian TGA also aligns with these timelines) are:
- 31 December 2027 for Class 4 IVDs,
- 31 December 2028 for Class 3 IVDs, and
- 31 December 2029 for Class 2 IVDs.
- Source: TGA In Vitro Diagnostic Medical Device Guidance
Manufacturers of all medical device types are expected to comply with the EU MDR & IVDR.
- Manufacturers for medical devices being manufactured, imported or distributed in the European Union are expected to be in full compliance with the EU Medical Device Regulations (EU MDR) and In Vitro Diagnostic Medical Device Regulations (IVDR) subject to transition periods. These new EU regulations replaced previous EU Directives for Medical Devices and in vitro diagnostic (IVD) medical devices.
The EU MDR & IVDR now apply to all types of medical devices in all product classes, including medical software.
EU MDR & IVDR vs previous directives (MDD, AIMD, and IVDD)
What changed from the MDD, AIMD, and IVDD (EU Medical Device Directives) to the requirements in the EU MDR?
- The EU MDR (Regulation 2017/745) represents a significant expansion from earlier medical device directives
- There are now over 23 “General Safety & Performance” requirements (refer to EU MDR, Annex I and related Annexes)
- Many products were reclassified into higher-risk categories that require:
- The involvement of a Notified Body (NB) for Conformity Assessments for Conformity Certificates/CE marks
- The involvement of expert panels for assessments of technical documents & clinical evaluations for high-risk products
- More robust clinical evidence and assessments of clinical evaluations, continuing across the product’s life cycle
- The expansion of the EUDAMED database is ongoing, and in a few years, it will be a requirement for Manufacturers to enter their product data and UDI assignments into EUDAMED (including the Basic UDI, UDI-DIs, and UDI-PIs)
You’ll learn more about EU regulatory changes in this cGMP eLearning course, covering the updated EU MDRs. This course generally takes from 2 to 4 hours to complete to gain your Course Certificate.
What should Manufacturers, Importers and Authorised Representatives understand about the EU MDR?
- The overall focus of the EU MDR is on ensuring more robust risk-management activities related to:
- Clinical Evaluation requirements (Clinical Evaluation reporting requirements)
- Additional post-marketing surveillance responsibilities
- Quality Management System (QMS) requirements (generally aligned with ISO 13485)
- Risk Management Requirements (generally aligned with ISO 14971)
- Joint liability for product defects (the manufacturer is not solely liable, and Authorised Representatives are jointly liable)
This course helps EU medical device manufacturers, importers, and other supply chain stakeholders understand their responsibilities and liabilities for medical device product safety and performance. A risk-based approach must be maintained across the entire life cycle of the medical device.

Quality Risk Management (QRM)
Quality Risk Management must be implemented across the entire life cycle of a medical device. Risk management activities, discussed in the EU MDR & IVD training presentation, must continue across the entire life cycle of a medical device or in vitro diagnostic product (IVD), in order to ensure product safety and performance.
This includes production and post-production monitoring of product safety information and updating EUDAMED (the European Union’s Database/Databank for Medical Devices).
Learn what Regulatory Authorities expect for EU MDR & IVDR audits/site inspections
By completing this EU MDR & IVDR certificate training course, you will gain insight into what Regulatory Authorities expect in terms of the application of risk management principles to medical devices, across the life cycle of a medical device, including for IVDs and medical software.
EU MDR – cGMP Course Certificate
cGMP eLearning
Recommended blended learning
cGMP eLearning courses, including this EU MDR presentation, are best combined with ongoing:
- Onsite training
- Product-specific training
- Supervision
- Mentoring
cGMP Education Purposes Only
Online GMP courses are for education purposes only. Such information is never to be used for operational decision-making.
Learners will still need to read, understand, and comply with the EU’s medical device regulations (MDR) & in vitro medical device regulations (IVDR) as applicable to their product and distribution regions.
However, the benefits of training include information relating to auditing expectations and EU regulations for medical device manufacturers.
Did you know…the updated EU MDR now comprises 175 pages?
- Many individuals do best by following the sequence below:
- Reading the EU MDR & IVDR regulations as applicable to their regions/product lines
- Taking the online training course including the online Assessment for the EU MDR & IVD Certificate of Completion for the course
- Re-reading the regulations again (as often as necessary to gain a thorough understanding of the requirements)
- Revisiting this course more than once is permitted and may benefit some learners (12 months’ access is provided upon purchase)
- Completing the required courses for QMS and risk management (ISO 13475 and ISO 14971)
- Creating a checklist for compliance with the EU MDR & EU IVDR requirements
- Following the regulatory requirements and documenting all related activities including compiling the technical dossier and continuing clinical evaluations across the product’s life cycle (via post-market surveillance activities)
You can find the EU MDR and EU IVDR on the European Commission’s (EU MDR) website.
Disclaimer:
- Regulatory training reinforces a compliance culture and reminds employees of essential QMS requirements and recordkeeping requirements
- Regulatory compliance courses are not intended to replace onsite, product-specific training and must never be used for decision-making purposes
- Learners should thus also be trained to follow all relevant accepted standards (e.g. ISO standards) & GMP compliance requirements relevant to their medical devices according to:
- Their medical device classification (risk class)
- Their distribution regions/jurisdictions
- Their roles and responsibilities
Resources: EU Medical device regulations and guidance notes

Download the PDF of the EU MDR (Regulation 2017/745).
Review other online training courses for the Medical Device manufacturing industry.
Earn your GMP training certificate in “EU MDR & IVDR ” to add to other GMP education topics including deviations management, complaints management, and CAPA system requirements!
Note: You can purchase several courses in a discounted training bundle – (consider combining 4 or more courses in a training bundle — up to 20 courses — to save on your GMP regulatory compliance education expenses).
Bundled training options let you ‘mix and match’ standard GMP compliance training courses and/or assign courses to different employees when required (enabling ‘just in time’ GMP training).
Pre-requisites for EU MDR training
- There are no formal pre-requisites for this course.
- However, it would be beneficial for you to complete the following two training courses prior to completing the EU MDR risk management course so that you understand globally accepted standards relevant to the Medical Device Sector/Industry.
- These medical industry standards include but are not limited to:
- Other relevant courses include:
- Complaints Management course
- Deviations and Non-Conformances management course,
- CAPA systems training course
- Data Integrity training course
Public training (Facilitated).
Click here for a list of face-to-face courses delivered via Zoom (onsite training of large groups of personnel may also be available, where feasible).
Explore the online GMP special training bundles (combined training packages).

Purchase a single cGMP training course or purchase a few of the courses you need, via a custom training bundle.
- Note: one learner, one cGMP course completion Certificate per course purchase.
- Learners will have 12 months’ access to the course.
- Learners will gain a downloadable Certificate of Completion for this education topic upon successful completion of the online assessment for this cGMP education topic.
All online course prices are in USD.
Conversion example: $40 USD = approximately $60 AUD but can vary slightly depending on changes to the US/AUD exchange rate.
All successfully completed courses provide learners with a time-dated GMP Training Certificate for successful completion of that particular GMP education topic.
Review other top-ranked GMP courses and best-practice GMP training courses.
How to order this course.
- Add the course to your basket and complete your purchase.
- Remember, the course fees are listed in US Dollars.
- Check your emails (all folders) for log-in instructions approximately 5 to 10 minutes after your order is finalised.
- Be sure to search for/add the following domains to your safe sender’s list: “@onlinegmptraining.com” and @pharmout.net”.
- This EU MDR & IVDR compliance course is available fully online (24/7 access) for 12 months from date of purchase.
- One learner (user) per licence.
cGMP Certificates of Completion
- Completion of any of our online GMP courses will enable you to print your GMP topic Course Certificate.
- DO DOWNLOAD your dated certificate on completion.
- You can attach your Certificate of Completion to your CV when applying for jobs in the pharmaceutical sector or when asking for a role change/career promotion.
FAQ: Does your medical software product need to comply with the EU MDR?
- If your product is being used in the European Union, it is likely your software needs to comply fully with the EU MDR and IVDR (EU medical device Regulations 2017/745 & 2017/746, respectively).
Here’s a resource for finding out if your software must comply with the EU MDR/IVDR requirements:
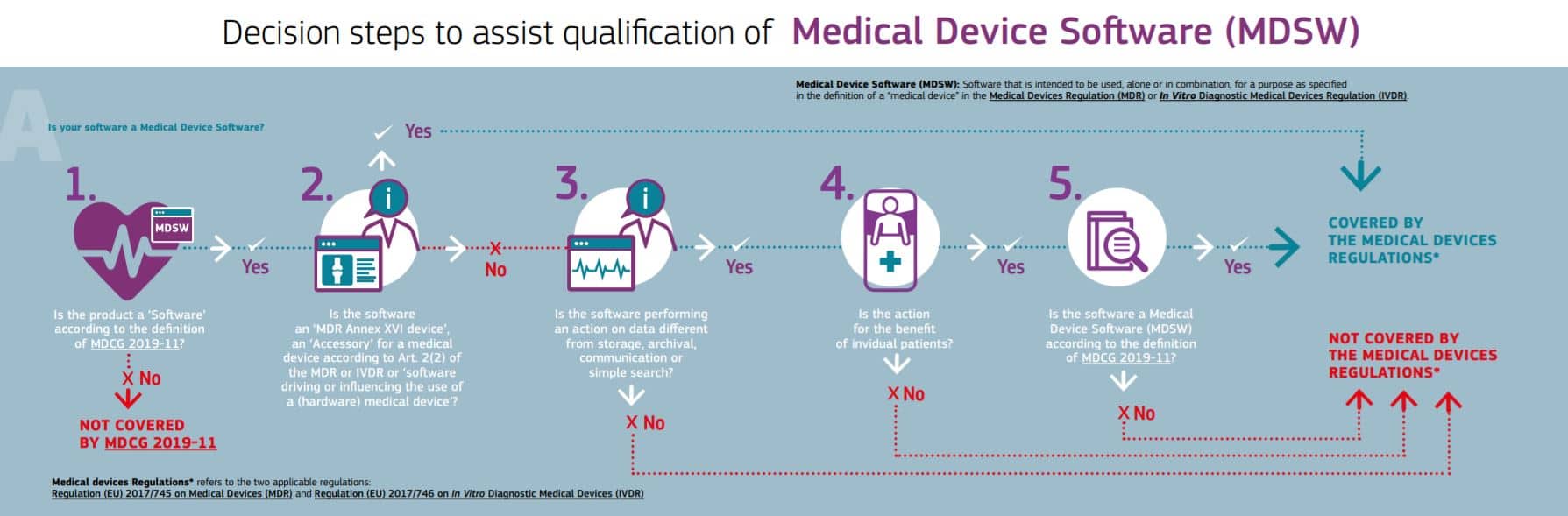
Source: European Commission (EC): Is your software a medical device?
If applicable to your software/medical device, learn more about medical software regulatory compliance requirements in the SaMD (Software as a Medical Device) online training course. This course is suitable for a general global audience.
Only logged in customers who have purchased this product may write a review.









Reviews
There are no reviews yet.