Understanding cGMP Certification
For therapeutic goods to reach patients and consumers, they must be shown to meet Good Manufacturing Practices (GMP)….

For therapeutic goods to reach patients and consumers, they must be shown to meet Good Manufacturing Practices (GMP)….

Did you know you can mix and match GMP education topics when ordering online GMP training courses? You…

FAQs about Software as a Medical Device (SaMD) Medical-purpose software development is a rapidly growing industry. As with…

In our latest Online Learning Trends (2020) article – Part 2: Virtual Reality Trends, we noted the increasing…

Is hosting a webinar better than creating online training courses (e-Learning modules)? Should you host a webinar series…

Good documentation practices (GDocP or GRK) are a crucial component of the Pharmaceutical Quality System (PQS) or Quality…
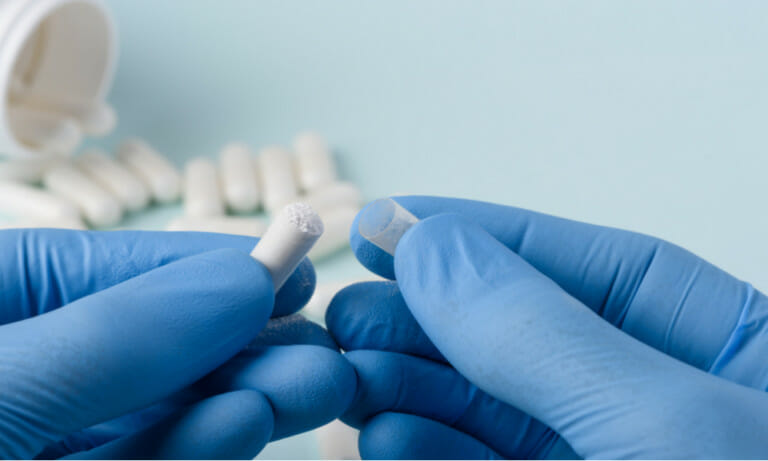
Do you have Pharmaceutical employees who need EU cGMP training, including validation, recordkeeping (GDocP) and GDP? Or perhaps…
End of content
End of content